Understanding Phosphorus Crystallization
Phosphorus is a fascinating element that plays a crucial role in various fields, from agriculture to materials science. One of the most intriguing aspects of phosphorus is its ability to form crystals under specific conditions. In this article, we will delve into the world of phosphorus crystallization, exploring its mechanisms, properties, and applications.
The Basics of Phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. It is a nonmetal that belongs to the nitrogen family (Group 15) of the periodic table. Phosphorus exists in several allotropic forms, including white, red, and black phosphorus, each with distinct properties and crystal structures.
White Phosphorus
White phosphorus (P4) is the most reactive and unstable allotrope of phosphorus. It consists of tetrahedral P4 molecules arranged in a cubic crystal structure. White phosphorus is highly flammable and emits a characteristic garlic-like odor. Due to its reactivity, white phosphorus is rarely found in nature and is primarily used in the production of other phosphorus compounds.
Red Phosphorus
Red phosphorus is a more stable allotrope compared to white phosphorus. It is obtained by heating white phosphorus to around 250°C in the absence of air. Red phosphorus has a polymeric structure, consisting of chains of phosphorus atoms. It is less reactive than white phosphorus and has a higher melting point (around 590°C). Red phosphorus is commonly used in the production of matches and as a flame retardant.
Black Phosphorus
Black phosphorus is the most stable allotrope of phosphorus. It has a layered structure similar to that of graphite, with each layer composed of phosphorus atoms arranged in a honeycomb pattern. Black phosphorus is a semiconductor with unique electronic and optical properties, making it a promising material for various applications, such as electronics and energy storage.
The Crystallization Process
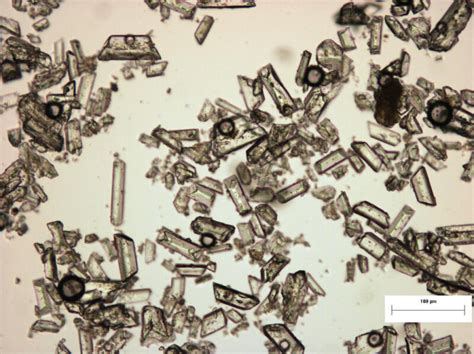
Phosphorus crystallization involves the formation of solid crystals from a liquid or vapor phase. The process is governed by thermodynamic and kinetic factors, including temperature, pressure, and supersaturation.
Nucleation
Nucleation is the initial stage of crystallization, where small clusters of atoms or molecules (nuclei) form in the liquid or vapor phase. These nuclei serve as the foundation for crystal growth. Nucleation can be homogeneous (occurring spontaneously) or heterogeneous (induced by foreign particles or surfaces).
Crystal Growth
Once stable nuclei are formed, they continue to grow by the addition of atoms or molecules from the surrounding medium. The growth process is influenced by factors such as temperature, supersaturation, and the presence of impurities. Crystal growth can occur through various mechanisms, including:
- Layer-by-layer growth: Atoms or molecules attach to the crystal surface, forming complete layers.
- Spiral growth: Atoms or molecules attach to the crystal surface at screw dislocations, resulting in a spiral growth pattern.
- Dendritic growth: Rapid and irregular growth occurs along specific crystallographic directions, forming tree-like structures.
Polymorphism
Polymorphism refers to the ability of a substance to exist in different crystalline forms. Phosphorus exhibits polymorphism, as evidenced by its various allotropes (white, red, and black phosphorus). Each polymorph has a unique crystal structure and properties, which can be influenced by the crystallization conditions.
Factors Affecting Phosphorus Crystallization
Several factors can influence the crystallization of phosphorus, including:
Temperature
Temperature plays a crucial role in phosphorus crystallization. Different allotropes of phosphorus are stable at specific temperature ranges. For example, white phosphorus is stable at room temperature, while red phosphorus is formed by heating white phosphorus to around 250°C. Black phosphorus is obtained at even higher temperatures (above 500°C) and pressures.
Pressure
Pressure can also affect phosphorus crystallization. High pressures favor the formation of denser allotropes, such as black phosphorus. The application of pressure during crystallization can lead to the formation of unique crystal structures with novel properties.
Supersaturation
Supersaturation refers to a state where the concentration of a substance in a solution exceeds its equilibrium solubility. In the case of phosphorus crystallization, supersaturation can be achieved by cooling a saturated solution or by evaporating the solvent. Higher levels of supersaturation promote faster nucleation and crystal growth rates.
Impurities
Impurities can significantly impact phosphorus crystallization. They can act as nucleation sites, promoting heterogeneous nucleation and influencing the crystal growth process. Impurities can also be incorporated into the crystal structure, altering its properties and stability.
Applications of Phosphorus Crystals
Phosphorus crystals have found various applications in different fields, including:
Semiconductors
Black phosphorus has emerged as a promising semiconductor material due to its unique electronic and optical properties. Its layered structure and tunable band gap make it suitable for applications in transistors, photodetectors, and solar cells.
Flame Retardants
Red phosphorus is widely used as a flame retardant in plastics, textiles, and other materials. Its ability to release phosphoric acid upon heating helps to inhibit the combustion process and prevent the spread of flames.
Matches
Red phosphorus is a key component in the production of safety matches. It is used in the striking surface of the matchbox, where it ignites upon friction with the match head containing potassium chlorate.
Fertilizers
Phosphorus is an essential nutrient for plant growth and is commonly used in fertilizers. Phosphate rock, which contains crystalline phosphate minerals, is mined and processed to produce phosphate fertilizers.
Challenges and Future Directions
Despite the progress made in understanding phosphorus crystallization, several challenges remain:
- Controlling the crystallization process to obtain desired crystal structures and properties.
- Scaling up the production of phosphorus crystals for industrial applications.
- Exploring new allotropes and polymorphs of phosphorus with novel properties.
- Developing environmentally friendly and sustainable methods for phosphorus crystallization.
Future research in phosphorus crystallization will focus on addressing these challenges and unlocking the full potential of phosphorus crystals in various applications.
Frequently Asked Questions (FAQ)
-
What is phosphorus crystallization?
Phosphorus crystallization is the process by which solid phosphorus crystals are formed from a liquid or vapor phase under specific conditions of temperature, pressure, and supersaturation. -
What are the different allotropes of phosphorus?
The main allotropes of phosphorus are white phosphorus (P4), red phosphorus, and black phosphorus. Each allotrope has a distinct crystal structure and properties. -
How does temperature affect phosphorus crystallization?
Temperature plays a crucial role in phosphorus crystallization. Different allotropes of phosphorus are stable at specific temperature ranges. For example, white phosphorus is stable at room temperature, while red phosphorus is formed by heating white phosphorus to around 250°C. -
What is the role of impurities in phosphorus crystallization?
Impurities can significantly impact phosphorus crystallization. They can act as nucleation sites, promoting heterogeneous nucleation and influencing the crystal growth process. Impurities can also be incorporated into the crystal structure, altering its properties and stability. -
What are some applications of phosphorus crystals?
Phosphorus crystals have found various applications in different fields, including semiconductors (black phosphorus), flame retardants (red phosphorus), matches (red phosphorus), and fertilizers (phosphate minerals).
| Allotrope | Crystal Structure | Stability | Formation Temperature | Applications |
|---|---|---|---|---|
| White Phosphorus | Cubic | Unstable, highly reactive | Room temperature | Production of phosphorus compounds |
| Red Phosphorus | Polymeric chains | More stable than white | Around 250°C | Matches, flame retardants |
| Black Phosphorus | Layered | Most stable allotrope | Above 500°C | Semiconductors, electronics |
In conclusion, phosphorus crystallization is a fascinating process that gives rise to various allotropes with unique properties and applications. Understanding the mechanisms and factors governing phosphorus crystallization is crucial for harnessing its potential in fields ranging from materials science to agriculture. As research in this area continues to advance, we can expect new discoveries and innovations that will further expand the utility of phosphorus crystals.



Leave a Reply